Date
2021/02/17
Duration
5 min read
ebook
Soil Quality: 5 Soil Biology
Organisations
SoilsWest
Department of Primary Industries and Regional Development
Grains Research and Development Corporation
University of Western Australia
What is rhizoctonia root rot?
Rhizoctonia root rot (also called rhizoctonia bare patch) is caused by Rhizoctonia solani AG8, a fungus that survives on crop residues and soil organic matter and is adapted to dry conditions and lower fertility soil. This disease is often a problem in sandy or calcareous soil of western and southern Australia. However, with the increased adoption of minimum tillage and intensive cereal cropping, it now occurs in a wider range of environments from Western Australia to southern New South Wales.
Rhizoctonia root rot can cause yield loss in a range of crops including cereals, pulses and pastures with inoculum levels increasing most rapidly on cereals and grasses. Of the cereals, oat is most tolerant followed by triticale and then wheat. Barley is most susceptible. The fungus causes crop damage by pruning newly emerged roots (spear-tipped roots) and can occur from emergence through to crop maturity. The infection results in plant stress, as the roots have been compromised in their ability to translocate both water and nutrients. Rhizoctonia root rot can cause uneven crop growth and damage to both crown and seminal roots, impacting crop yields. For example, wheat grain loss of 8–19% and, in barley, grain loss as high as 25%, commonly occur in heavily infested paddocks in Western Australia.
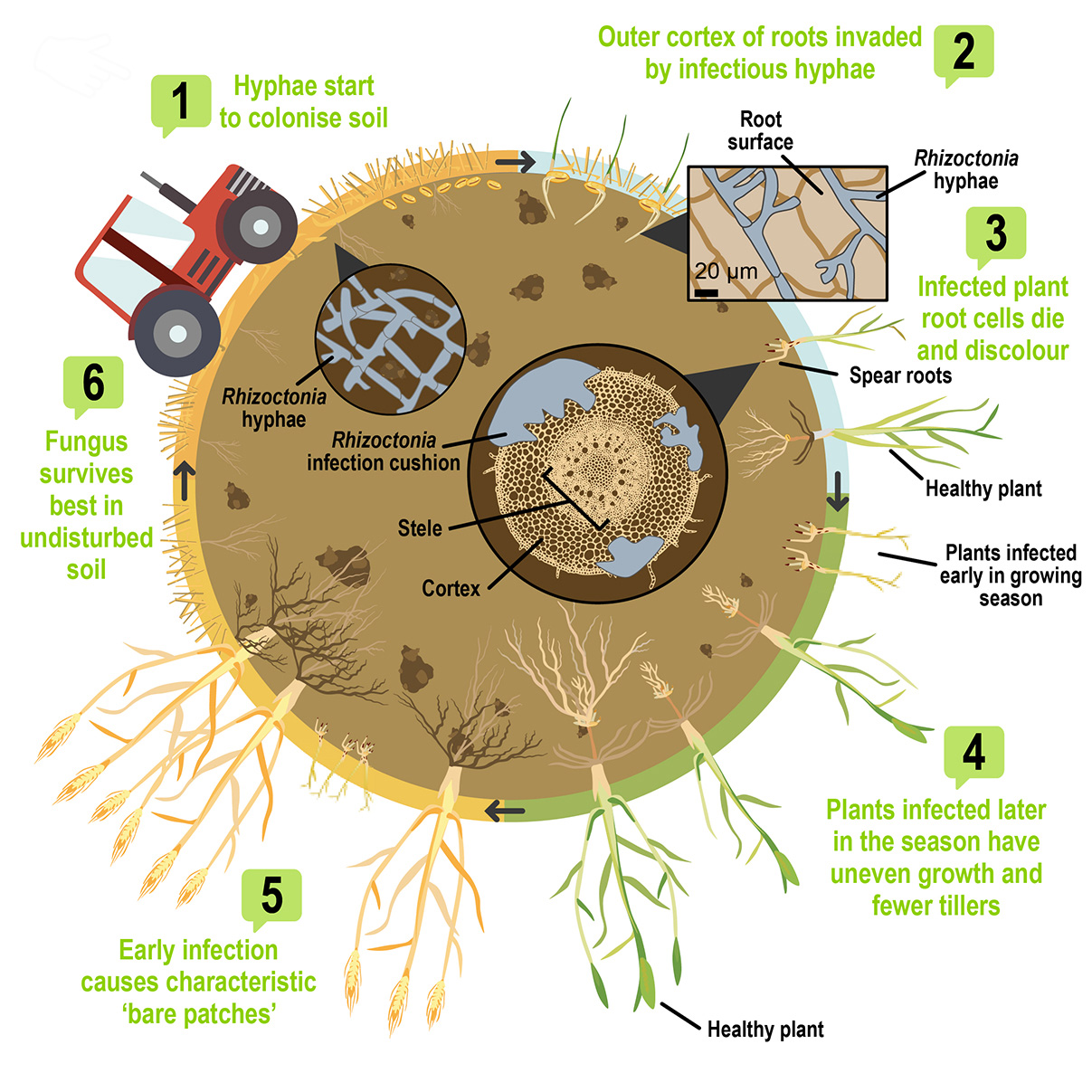
Symptoms and management of rhizoctonia root rot
Severe infection by R. solani at the seedling stage causes patches of poor crop growth from very small areas to several metres across. This can occur in cold and dry soil and other conditions that restrict seminal root growth, for example, compaction, lack of moisture or herbicide residues. Plants within these patches will be stunted and often have yellow leaves. If infection has occurred early and conditions are dry, plants may die. Warmer soil temperatures and adequate moisture are less conducive to the disease as crops with early vigour escape seminal root infection. Unfortunately, crown roots, which form later, can be affected in infested paddocks and still cause significant yield losses. This type of infection leads to symptomatic uneven growth during tillering.
The widespread adoption of minimum-tillage farming has resulted in conditions more favourable to the disease. Without tillage, the hyphal network developed by the fungus can remain intact and allows easier spread once conditions are favourable. In cereals, the inoculum builds up from sowing and generally peaks at crop maturity. For other, broad-leaf crops such as legumes and canola, inoculum will generally decline. Crown root infection late into the crop season results in the build-up of inoculum in cereal crops, while rain post-maturity of a crop and fallow over the summer causes a decline in inoculum.
Effective control of rhizoctonia root rot in cereal crops requires sound management practices spread over more than one cropping season. In the absence of host plants, including weeds, summer rainfall events of more than 20 millimetres in a week can substantially reduce the level of inoculum. Dry spells, on the other hand, offer little opportunity for stubble breakdown, with disease levels likely to remain stable if a host, or stubble, are present.
The disease is an ongoing problem in the low to medium rainfall areas of the south-western agricultural region of Western Australia, especially on sandy soil, and in some areas of the high rainfall zone. While R. solani is likely to be present in many soil types, it is not necessarily going to cause a problem. In some soil, beneficial soil microorganisms and high microbial activity have been shown to suppress the expression of the disease and reduce the level of disease. Building these beneficial soil microbial communities takes time. For example, in some cropping systems with stubble retention, suppressive activity has been shown to increase over a five- to eight-year period.
References
ebook Soil Quality: 5 Soil Biology
Murphy D, Hoyle F, Collins S, Hüberli D, and Gleeson D (2021).