By definition, pollutants are organic or inorganic substances present in harmful amounts (above background concentrations) within air, water and soil.
Land contamination is defined as land that has present a pollutant (or pollutants) at above-background concentrations causing, or with the potential to cause adverse impacts to human health, the environment, or any environmental value.
The primary causes of land and water contamination are often a result of poor or inadequate management practices linked to human habitation and development. Industry, mining, forestry, intensive agriculture (e.g. feedlots, horticulture, broadacre farming), roadworks and urban development all have the potential to pollute soils and waterways when poorly managed.
What are soil and water pollutants?
Pollutants can be organic or inorganic substances present in harmful amounts (above background concentrations) in the atmosphere, water, and in or on soil. In agriculture, ‘pollutants’ such as heavy metals, pesticides, herbicides, nutrients, eroded soil, oils, hydrocarbons, plastics and other potentially toxic chemicals are often introduced to the system through direct application, or through transport of these contaminants by wind, water or product. More broadly, the main causes of land and water contamination are poor or inadequate practices linked with human habitation and development.
The toxicity and persistence of these pollutants, as well as their direct uptake by people, plants and animals influence the potential impact on ecosystem and human health. Some pollutants have significant effects even in very low concentrations in water, and their detection and measurement are difficult. Persistent organic pollutants such as organochlorine insecticides and dioxins are fat-soluble, can spread widely in the environment and can move through the food chain to be concentrated at the top of the food chain. Inorganic pollutants like cadmium, lead, arsenic and mercury generally have a more localised impact.
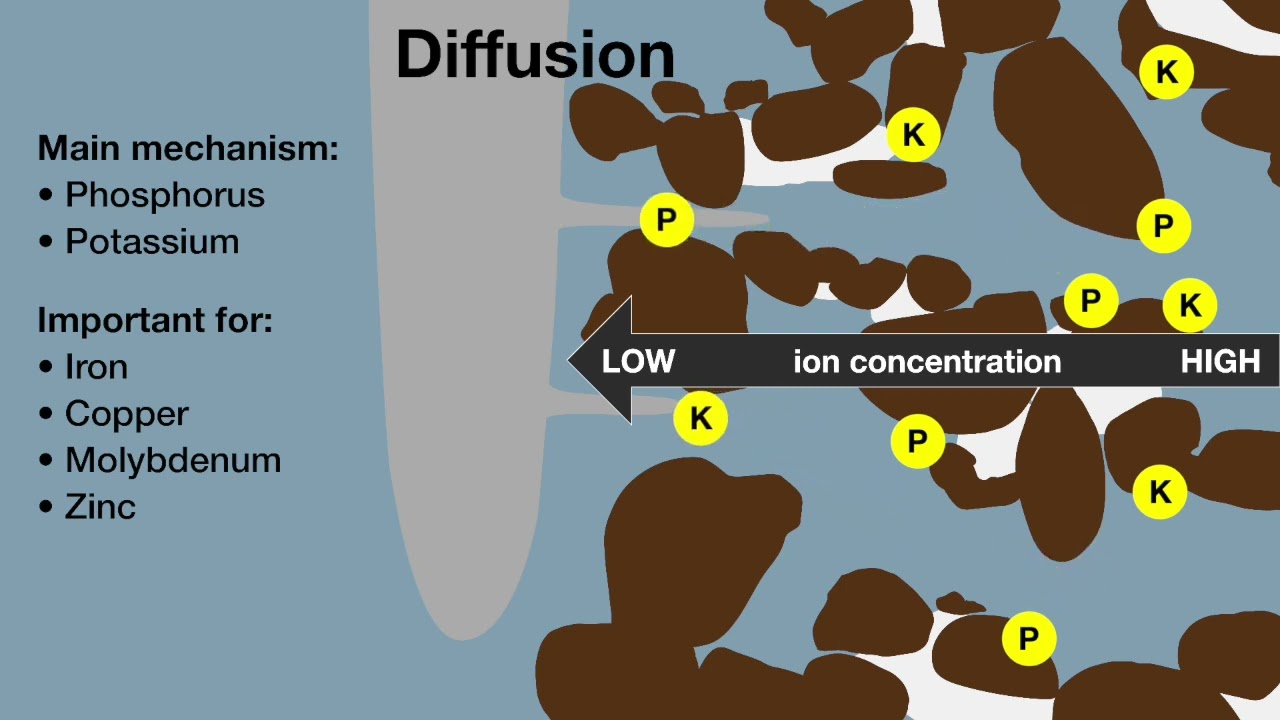
Point source (PS) pollutants enter into a system from a single point, whilst non-point source (NPS) or diffuse pollutants are more difficult to trace and control as they have multiple sources. Examples of point source pollutants include dairy waste from milking or stand-off areas, manure and slurry from intensive animal industries, sewage effluent drains, chemical and petroleum products leaking from storages. Non-point source pollutants include fertilisers and pesticides that are commonly applied by households, agriculture and broader industries. Accumulation of pollutants is often the result of a combination of factors – for example, accumulation of organochlorine in grazing animals is influenced by soil type, seasonal conditions, pasture composition, plant root structure, period of exposure, contaminant present and level of contamination.
Features of soil contaminated by pollutants
-
Presence of synthetic chemicals or their residues in soil, water or air, or other chemicals at above background levels
-
Decreased biological diversity and activity. Biological activity of specific organisms utilising the pollutant as a substrate can increase.
-
Poor plant growth and possible death. Potential contamination in grain and fruit.
-
Sickness or death of fauna/stock. Possible contamination of by-products.
-
Negative impacts on human health (through both direct and indirect consumption)
What factors influence the persistence of pollutants in the environment?
Agricultural activities that cause non-point source pollution include confined animal facilities, grazing, cultivation, pesticide spraying, irrigation and fertilisation – with contaminants including (but not confined to) sediment load, nutrients, pathogens, pesticides and salts. Point source pollutants usually arise from storage areas, spillages or intensive animal facilities. Impacts on surface water, ground water and surrounding habitats can be minimised by properly managing activities associated with non-point source pollution, and avoidance of point source pollution.
Organic chemicals added to soil are broken down in a similar way to organic matter and degraded by microorganisms that gain energy and carbon from the oxidation of the chemical structure. The ease with which a chemical is degraded depends on the complexity of its structure, as well as the frequency of exposure. Complex structures are often more difficult to degrade because fewer microorganisms in the soil produce enzymes capable of degrading them. However, soil microorganisms can adapt when a chemical is added to soil repeatedly. Microorganisms capable of degrading the chemical grow and multiply, resulting in more rapid breakdown of further applications (with loss of persistence or efficacy). This may be a problem when a pesticide is degraded too quickly, preventing it from killing the pest – but can also be a solution when cultured specifically and applied to treat spills.
Chemical degradation rates within soil are affected by temperature, whether the pollutant is water soluble, soil reactions and management practices. For example, the herbicide atrazine can leach from soil into waterways, whereas the herbicide glyphosate is strongly adsorbed, rendering it inactive once in contact with soil. Decomposition half-lives (or the time taken for a substance to reduce to 50% of its initial value) can vary from days to years for different chemicals.
In agriculture, the two primary nutrient pollutants are nitrogen (N, with nitrate being more mobile in the environment) and phosphorus (P). Eutrophication of freshwater bodies can occur when nitrogen and phosphorus enter waterways – primarily from surface water movement. While much of this nutrient load results from fertiliser applications, nitrate produced by the mineralisation of organic matter and stimulated by soil disturbance in autumn when there is little plant uptake may contribute to the overall surplus.
Sediments in surface water run-off which carry nutrients and chemicals primarily result from a lack of plant cover, soil erosion and mismanagement (e.g. poor timing of application). Water repellent soil with poor infiltration is also a contributor to poor water entry into the soil. Maintaining adequate ground surface cover, zero tillage and improving management of lands adjacent to waterways through buffer strips for example, can help reduce pollutants entering waterways and enhance water quality.
In Western Australia, nutrient pollution is more likely to occur in late autumn at the break of season prior to crop and pasture establishment, or during the winter dominant rainfall season. Pre-sowing or at-sowing fertiliser, can add to any nutrient release from the mineralisation of organic matter to increase soil nutrient levels where there is little or no plant uptake – increasing the risk of loss. Increased surface water runoff and leaching associated with winter rainfall can then mobilise these and other nutrients, impacting on the environment. This can lead to eutrophication of waterways, such as occurred in the Darling River causing a depletion of oxygen and resulting in significant ecological damage.
Cadmium which occurs naturally in soil, rock, water, plants and animals is also a contaminant of agricultural industries. Cadmium can accumulate in humans and at high levels, can adversely affect human health. Atmospheric cadmium is most likely to be high close to industrial activities such as smelting, with agricultural contaminants largely confined to some of the phosphorous containing fertilisers, trace element fertilisers and phosphogypsum. Concentrated phosphatic fertilisers currently used in Australia (i.e. diammonium phosphate – DAP; mono ammonium phosphate – MAP; triple superphosphate – TSP) are generally low in cadmium, whilst pasture grades of single superphosphate can be higher in cadmium. Check with your local fertiliser representatives for state standards.
Insecticides, herbicides, and other pesticides are common contaminants of soil, air and water. They can be mobilised by either wind or water flow and deposited in soils, waterways or groundwater resulting in both localised and off-site environmental impacts.
Spray drift from pesticide application can enter water courses if crops are located too close to the river and can contaminate adjacent crops and land, if the correct rate and application method is not used under appropriate weather conditions. The main health threat however comes, not from spraying, but from poor storage and accidental spillage.
Although phased out of use over a decade ago, the presence of persistent organic pollutants such as aldrin, dieldrin, and dichlorodiphenyltrichloroethane (DDT) can still be detected at contaminated sites, in sewage biosolids, and in the fat of fish and marine mammals.
In high rainfall areas – and particularly when plant cover is low during summer, increased run-off and drainage increases the risk of residual herbicides such as atrazine (which persists in soil) entering waterways or groundwater and affecting water quality. Similarly increased runoff and leaching associated with winter rainfall from fertilised paddocks can mobilise soil and nutrients, contributing to greater eutrophication of waterways. Increasing temperature accelerates the breakdown of organic compounds.

How do agricultural pollutants affect soil health?
Pollutants can result in poor plant emergence and growth (including mutations), as well as failure to flower and set seed. These may be direct effects on the plant, or indirect through effects on the soil biota. The impact can be both on-site and off-site dependent on the movement of the pollutant. There is evidence that where high levels of contaminants (e.g. cadmium) accumulate, they can be translocated into the food chain through plant material and grain – contributing to concerns for possible food contamination affecting both grazers and humans.
These pollutants, as well as some pesticides, may constrain future land use if contamination risk is severe. Examples that follow are specifically for waste by-products.
- Milk spillage from dairies is considered a serious environmental contaminant as it can accelerate the growth of bacteria. Large scale spills can be associated with the death of aquatic animals and undrinkable water in marine ecosystems.
- Undiluted animal manure (slurry) has been shown to be up to one hundred times more concentrated than domestic sewage. Manure slurry can carry a parasite capable of contaminating drinking water (Cryptosporidium) which is difficult to detect – making it unsafe to drink. Air pollution can also result from the releases of gases such as methane and hydrogen sulfide.
- Silage liquor (from fermented wet grass) is even stronger than slurry, with a very low pH and a very high biological oxygen demand. It can be highly corrosive, causing damage to storage equipment and accidental spillage.
- Polychlorinated biphenyls (PCBs), heavy metals, as well as endocrine hormones have been detected in sewage biosolids.
The effect of pesticides on soil microorganisms within the soil is dependent on the active constituent, mode of application and soil type. Negative effects are generally more common on sandy, coarser textured soil – though many of these impacts are determined to be transient in nature (short-term). Direct effects are generally observed within a season (i.e. on nutrient availability or toxicity), whilst indirect effects take longer to be observed (i.e. on organic matter and nutrient cycling).
Pesticides can contribute to a depreciation of the natural resource (through the use of non-renewable resources, loss of natural habitat, loss of biodiversity) associated with impact on non-target species.
Pollutants contaminate groundwater and waterways, and even at very low concentrations can negatively impact on lake and river systems (through algal blooms, eutrophication and ecological damage). The recreational, agricultural and industrial use of the water is impaired, and the safety of drinking water potentially reduced, all of which have significant implications for food and human health. Phosphorus and nitrogen are the main nutrients contributing to eutrophication of surface and groundwater, though pesticides are also a known contaminant. Sediment from soil erosion and organic wastes also contribute to a decline in water quality and ecosystem health.
Agricultural activities cause emissions of three greenhouse gases: carbon dioxide (CO2), nitrous oxide (N2O), and methane (CH4) – each with differing intensity in terms of impact on climate change. Nearly 13% of Australia’s total greenhouse gas emissions (549 Mt) come from agriculture – with a large contribution from methane associated with livestock (72%) and nitrous oxide (nearly 19%) from soil.
Soil is both a sink and a source of carbon emissions primarily linked to cycling of soil organic matter. Decomposition of soil organic matter releases carbon dioxide. This is primarily associated with conversion of land to cultivation, soil and residue management.
Low nutrient use efficiency in nitrogen-based fertilisers contributes to our emissions. Microbes in soil break down nitrogen fertilisers to produce nitrous oxide – a potent greenhouse gas that contributes to global warming. An example of where this occurs is under anaerobic conditions (waterlogged soil), where excess nitrate in the soil is converted into nitrous oxide.
Lead, arsenic, mercury, and cadmium (all main industrial pollutants) can accumulate in the human body over long periods of time, as they are difficult to flush out of our system. Some chlorinated organic chemicals and endocrine hormones are also either long-lasting or have significant effects on human health.

Managing soil and water pollutants
Monitor product quality, stock health, and river water quality.
Many agrochemicals are broken down by soil biota and their effectiveness will be modified by the soil environment. Herbicide degradation is faster in soils with high microbial activity. Toxic elements such as arsenic can be ‘locked’ up in the soil by microorganisms, preventing further pollution. Certain plants are able to concentrate specific contaminants, including many of the heavy metals. Over time the soil becomes depleted in the element, but the plant material must then be managed carefully
The economic basis for introducing contaminant degrading bacteria is likely to vary depending on the contaminant and value of the industry. For example, it can be a relatively inexpensive and extremely effective method of cleaning up oil spills, but can be very slow.
Synthetic pyrethroids and organophosphates have replaced more toxic and persistent pesticides used several decades ago and have considerably less impact in the environment. Use these chemicals only at recommended rates and for registered purposes, and practice integrated pest and weed management to reduce reliance on chemicals. Reduce chloride additions to soils through the use of irrigation water and fertilisers with low chloride concentrations to reduce cadmium contamination.
Identify areas contributing the bulk of harmful nutrients to the environment, and reduce nutrient loss through site-specific management. Work on the basis of replacement strategies (put on what comes off) and match fertiliser applications to plant requirements to avoid overfilling the storage capacity of the soil. Manage the rate, frequency, timing and method of application of fertiliser to avoid run-off and leaching.
Manage soil to improve nutrient retention and consider using alternative fertilisers such as organic materials, coarse rock gypsum, and rock phosphates to slow nutrient release. Using soil and tissue tests to develop appropriate fertiliser management strategies and adding phosphorus to soil only when tests indicate a deficiency will reduce the risk of excessive cadmium accumulation.
Perennial plants/pastures minimise nutrient losses from leaching and improve nutrient uptake at greater depth than crop species. Manage subsoil constraints to decrease runoff and erosion, and remove constraints to root growth to improve water use efficiency.
Address the underlying causes of pollution. Ensure chemicals are stored and mixed in secure areas with a bund or other retention system in case of spills, and high-risk weather conditions avoided. Use spray-free buffer areas adjacent to streams and water bodies. Ensure safe disposal of unused chemicals from spray tanks and containers.
Assess irrigation water quality and implement strategies to minimise channel seepage and erosion; irrigation scheduling to minimise runoff.
Protect soil from erosion, organic matter and structure loss, and salinity by using soil conservation measures in conjunction with minimum tillage and crop rotation; manage recharge areas to reduce accessions to groundwater.
Avoid flow of flood waters or runoff directly into waterways – use diversion channels, restore and maintain native vegetation, and establish grassed buffer zones where runoff enters a water course so that contaminanted sediment drops from the flow before it enters the waterway.
Pollution from intensive animal industries can be limited by storing and managing facility wastewater and runoff with an appropriate waste management system. Overgrazing exposes soils and increases erosion, so adjust grazing intensity, keep livestock out of sensitive areas, and revegetate rangeland and pastureland to minimise the impact of stock on waterways.
Point source soil contamination can be diluted or sometimes washed out with water – however this succeeds in only transferring the contamination to a water phase and does not address issues related to safe disposal. Organic and microbial treatments are now available for some types of soil contamination.

PAGE REFERENCES AND ACKNOWLEDGEMENTS
Material on this page adapted from:
- Hoyle FC (2007). Soil Health Knowledge Bank.
- Soil Quality ebook series. SoilsWest, Perth, Western Australia.
Last updated July 2024.