Sodic and alkaline soil overview
Soil affected by sodicity and alkalinity has multiple chemical constraints that combine to limit crop yield potential.
Under the broad classification, sodic soil accounts for 57% of the 18 million hectares of arable soil in the Western Australian wheatbelt. In New South Wales, Victoria and Queensland, sodicity is present as 47%, 45% and 73%, respectively, of soil types and globally accounts for more than 500 million hectares. In the south-western agricultural region of Western Australia, this soil often presents as duplex (texture contrast), sand over clay, or gradational with exchangeable sodium, pH and salinity increasing with depth. Affected soil is generally fine textured with clay content greater than 20%.
Sodic and alkaline soil disperses when wet, decreasing water infiltration, water storage, crop root development and grain yield. Yield variability across seasons is greater compared with less constrained soil. Wheat yield losses on sodic soil are in the order of 30 to 60% when compared with unconstrained soil. This provides an estimated average cost of lost production to Western Australian agriculture of between $31 and $128 per hectare per year.

Alkaline soils: the essentials
An overview of the causes, implications and impact of alkalinity on soil structure and stability. From Soil Quality: 2 Integrated Soil Management (Pluske et al. 2018). Video talent: David Hall, DPIRD; video editing: Science with Style.
What is a sodic and alkaline soil
Sodic and alkaline soil is defined by texture and chemistry. In general, affected soil has a clay texture. Sodic soil has an exchangeable sodium percentage greater than 6 and most has pH between neutral and alkaline. The concentration of sodium accumulates over time from the weathering of parent material, aeolian accessions and rainfall. Sodium is adsorbed onto clay particles, leading to the collapse of soil structure via dispersion, which blocks soil pores – reducing water infiltration and increasing the density of surface and subsurface soil. Adding to the complexity of sodic soil are other constraints that include high pH and chemical toxicities, including boron. These constraints often occur simultaneously in sodic soil, producing a hostile environment for growing plants.
Soil structure and aggregate stability
Sodic and alkaline soil is often poorly structured due to its dispersive behaviour. Soil structure describes the arrangement of primary mineral particles (sand, silt and clay) to form aggregates. The size, shape and packing of aggregates determines soil porosity – and how readily water and air move, and plant roots grow, through the soil matrix.
When immersed in water, aggregates often break down into smaller particles. Initially particles will slake where the soil breaks down into micro-aggregates (<250 µm). Slaking results from escaping entrapped air disrupting aggregate bonds and is often associated with low organic matter levels.
The breakdown of micro-aggregates and the separation of clay platelets is known as dispersion and is associated with weak electrostatic bonds between clay surfaces. The dispersed particles, carried by water, fill and block the pores within the soil matrix. As a consequence, water and air movement is impeded, resulting in reduced water infiltration and water storage, and increased soil density and soil strength. The dispersed particles form dense layers within the soil profile.
Factors that affect clay dispersion
Clay dispersion occurs where the bonds between clay plates are weakened or broken. Factors that affect dispersion include clay type (mineralogy), the concentration and type of ions in solution and adsorbed onto clay surfaces, soil pH, and the degree of mechanical disturbance. Soil most likely to disperse has:
- illite, smectite and montmorillonite clay
- low salt concentration
- cations with more than 6% exchangeable sodium*
- pH greater than 8.5
- a high level of physical disturbance.
*A soil is defined as sodic when the exchangeable sodium percentage (ESP) > 6 but a soil with an ESP below 6 can disperse just as those with an ESP above 6 don’t necessarily disperse.
Clay mineralogy
Some clay minerals swell when wet and shrink when dry. As water is absorbed between the layers, the lattices expand (swell), weakening the electrostatic bonds. Due to their structure, some clay minerals (montmorillonite, smectite and illite) swell to a greater extent and are more likely to disperse than others (kaolinite). Kaolinite is the dominant clay type found in the south-western agricultural region of Western Australia.
Sodicity
The surfaces of clay platelets are mainly negatively charged. These surfaces are bound together by weak electrostatic bonds with cations (positively charged ions). The types of cations are important. The strength of the electrostatic bonds created by cations between clay surfaces decreases in the order: aluminium (Al3+) > calcium (Ca2+) > magnesium (Mg2+) > potassium (K+) > sodium (Na+).
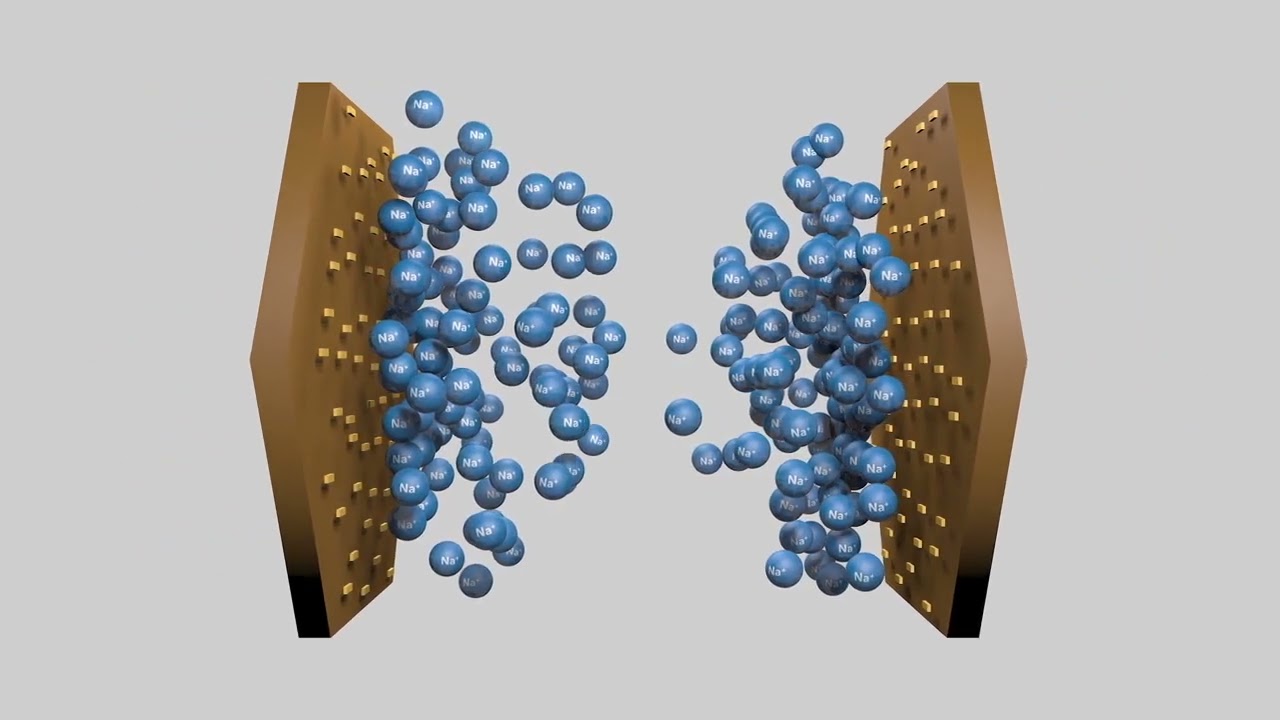
Dispersion of clay platelets by sodium
Sodium ions form weak bonds between clay platelets. Soil with low ionic strength and excessive sodium and magnesium is especially prone to dispersion. From Soil Quality: 8 Sodic and Alkaline Soil (Barrett-Lennard et al. 2022). Concept drawings: Ed Barrett-Lennard, DPIRD; animation: Red Empire; voice talent: Ed Barrett-Lennard, DPIRD.
Alkalinity
Soil pH can also affect soil dispersion. At neutral soil pH, clay platelets normally have negatively charged faces and positively charged edges. Clay platelets can therefore have ‘edge to face’ electrostatic attraction. When this occurs, the clays flocculate, they have high hydraulic conductivity, and salts are more easily leached. By contrast, at alkaline soil pH, the edge charges on clay plates become negative, there is electrostatic repulsion between the negatively charged platelet faces and edges, and the clays disperse. When this happens, the soil has very low hydraulic conductivity, it is non-leaching and salt accumulates in the soil profile.
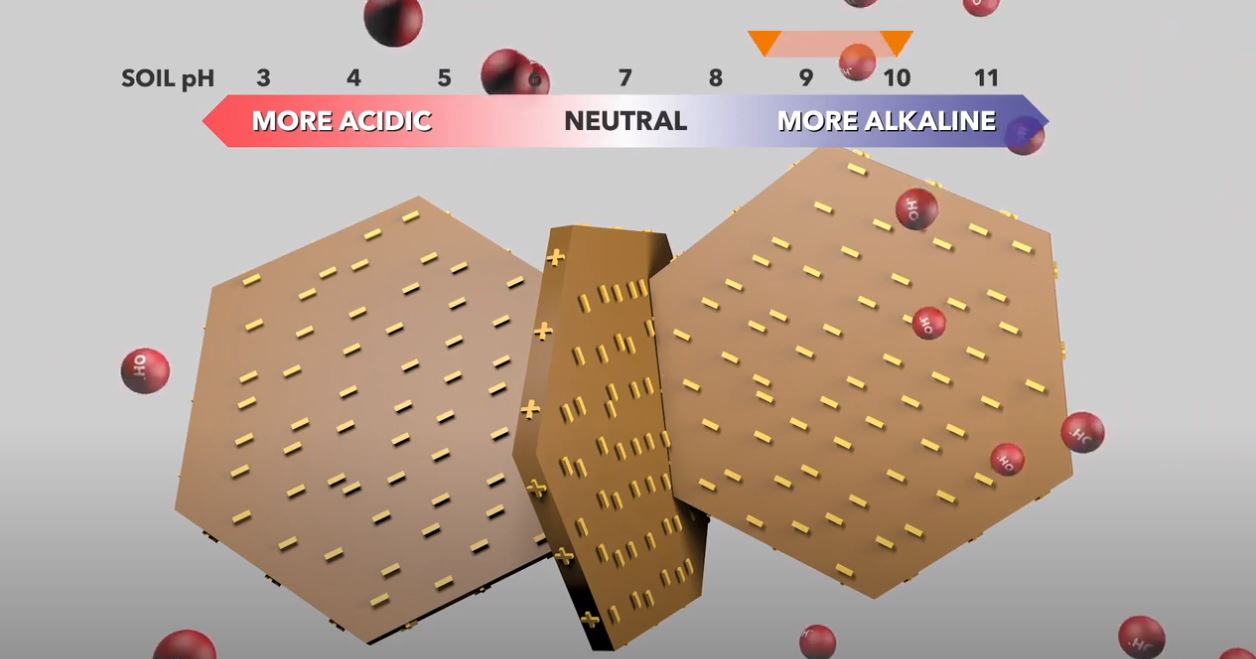
Dispersion of clay platelets by changing pH
From Soil Quality: 8 Sodic and Alkaline Soil (Barrett-Lennard et al. 2022). Concept drawings: Ed Barrett-Lennard, DPIRD; animation: Red Empire; voice talent: Ed Barrett-Lennard, DPIRD.
Soil disturbance
Soil disturbance including tillage, trampling by livestock and raindrop impact increase dispersion through the rearrangement of clay particles and the weakening of bonds. Trampling and tillage have resulted in a two- to ten-fold reduction in water infiltration due to increased slaking and dispersion.
Effects of aggregate instability and sodicity on transient salinity
One consequence of clay dispersion is the accumulation of salt. This can result in transient salinity, which is a common problem in sodic and alkaline clayey soil of semi-arid areas. Salt is generally present in small amounts in rainwater and also falls in aeolian dust.
Dispersed soil has low hydraulic conductivity and therefore salt cannot be readily leached. Salt can therefore accumulate in dispersive soil over hundreds of years. In wet years when soil is well-hydrated, there can be small amounts of leaching of this salt into the subsoil and the salinity of the soil solution is low – and not sufficient to impact on crop growth. By contrast, in dry years when soil is poorly hydrated, salts can be drawn upwards into surface soil layers by capillary action, and the salinity of the soil solution increases, becoming high enough to impact on the growth of crops. Elevated salinity of the soil solution slightly decreases plant available water but substantially decreases the plant’s ability to access that plant available water.
Impacts of sodic and alkaline soil
Sodic and alkaline soils in dry environments
Clay soil in semi-arid environments (250–350 mm average annual rainfall) is often affected by the combination of sodicity, alkalinity and transient salinity. As soil drys, water adheres to the fabric of the soil, making it less accessible to plants. This adhesion of water is greater for heavy textured soil (i.e. clay) than light textured soil (i.e. sand). As the amount of water in the soil decreases, solutes (such as salt) that are normally dissolved in the soil water become more concentrated in the soil solution. This causes osmotic stresses and ion toxicity effects on plants. These stresses both occur in sodic and alkaline soil, particularly in dry years. Both the adhesion of water to soil and increases in salinity of the soil solution decrease plant available water, as well as decreasing the accessibility of soil water to plants.
Highly dispersive soil has exceptionally poor leachability. This means that all substances (elements or chemicals) applied to the soil will accumulate in the subsoil. At high enough concentrations all of these can be toxic.
One element that can accumulate in the subsoil is boron. At low to moderate concentrations, boron is an important micronutrient and is essential to crop growth. At high concentrations, boron is toxic. Boron-toxicity produces characteristic brown spots on the leaves of susceptible crops such as barley. Toxic concentrations of boron may decrease the growth of susceptible crops, but these effects can be difficult to separate from the adverse effects of transient salinity.
Sodicity in wet and waterlogged environments
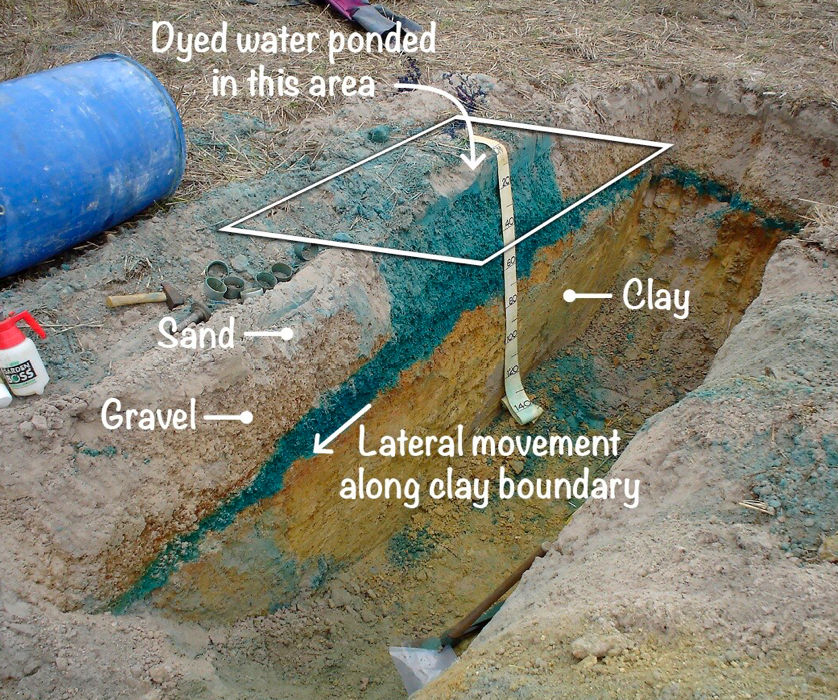
Restricted water movement in sodic soil affects not only the amount of water infiltrating and being stored in soil, but also the ability of the soil to drain.
Approximately 60% of soil in higher rainfall (>500 mm) parts of the south-western agricultural region are duplex, with permeable sands overlying relatively impermeable sodic clays. The differences in permeability between the two layers can be more than 10-fold.
In these soil types, water readily infiltrates through the sand layer but is prevented from draining due to the poorly structured subsoil clay. Water will begin to perch on top of this clay layer when rainfall exceeds evapotranspiration. If rainfall persists, the soil can become waterlogged (saturated). This perched water will either back-fill to the surface, or will move laterally along the sand-clay interface in sloping landscapes.
Crusts and hard layers
One consequence of dispersion in sodic and alkaline soil is the development of crusts and hard layers. Crusts form as the dispersed sediments settle, with the heavier sand fraction at the base of the crust, then silt and the fine clay fraction on the surface. Crust are commonly only a few millimetres thick and can be highly impermeable to water and air. Crusting can adversely affect germination and seeds trapped below crusts can rot.
Structural breakdown due to slaking and dispersion is also responsible for the development of hard-set layers that may be hundreds of millimetres thick. These layers are apedal – they cannot be penetrated when dry but can be penetrated when wet. The loss of structure restricts water entry and as the soil dries, soil strength increases to the extent that seedlings fail to emerge and root growth is severely restricted. Hardsetting is confined to soils with a loam texture that do not have sufficient swelling clay to crack open when dry and are inherently unstable when wet. Most clay and loamy textured soils within the eastern wheatbelt of Western Australia are prone to hardsetting.
Nutrient availability
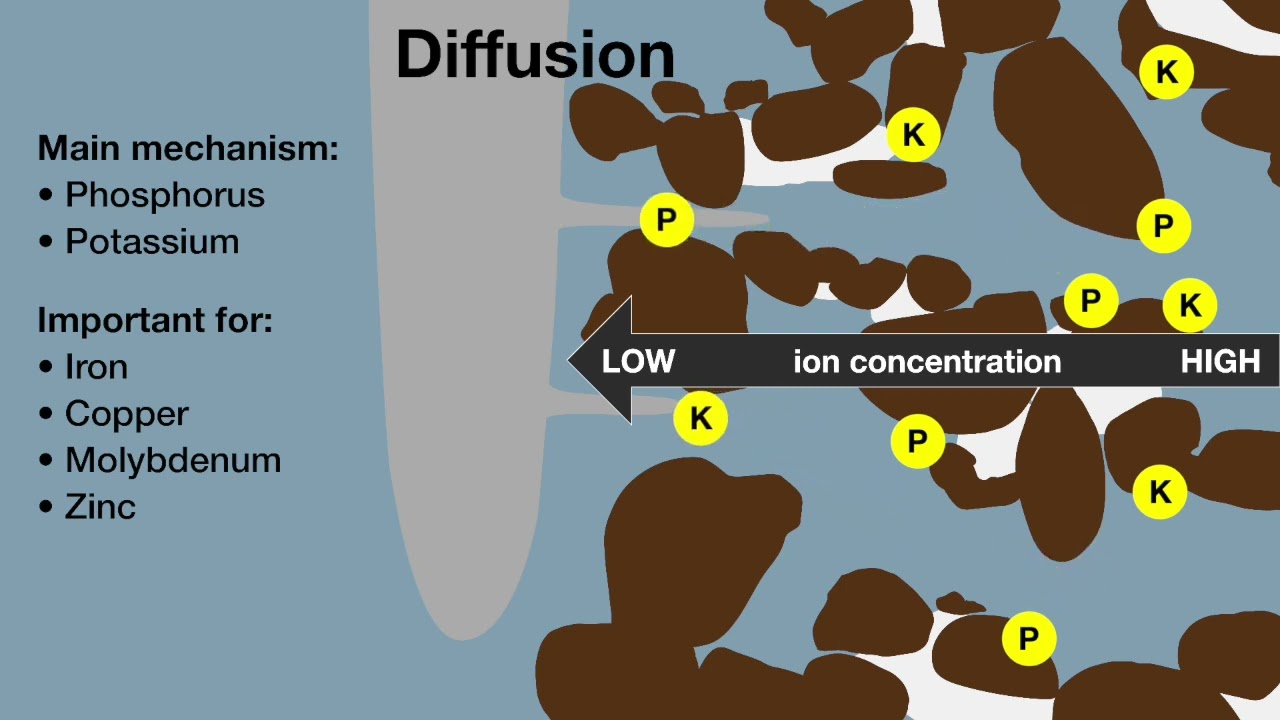
Many soil types, particularly in the more arid regions of the wheatbelt, are sodic and also have alkaline pH. Soil pH affects the types of ions in solution, the compounds formed, their solubility and hence their availability.
In moderately to highly alkaline soil (pHW >8) micronutrients, including iron (Fe), manganese (Mn), zinc (Zn) and copper (Cu) become less available to plants because the ionic compounds they form at higher pH have decreased solubility compared to those formed at lower pH. Of the major nutrients, calcium (Ca), magnesium (Mg) and nitrogen (N) are also less available.

Diagnosing sodic and alkaline soil
There are no hard and fast rules for diagnosing soil affected by sodicity in semi-arid and higher rainfall landscapes. At the moment protocols are still developing. These are our current best suggestions.
- Note the major vegetation forms in the area
- Dig a hole – Note the texture. Sodic and alkaline soil often has a sandy clay texture. Note the presence of calcareous nodules in the soil. Alkaline soil may be strongly calcareous at depths greater than 0.4 m. Collect soil samples at differing depths through the soil profile particularly where there are boundaries (soil layers) defined by texture, colour and carbonates.
- Test soil dispersion, sodicity (exchangeable cations), pH, EC1:5 and boron.
- Do an EM survey (if calcareous, alkaline and with elevated EC1:5)
Soil and landscape observations
Native vegetation
Soil affected by sodicity and alkalinity are often characterised by the principle trees making up the native vegetation. The original trees may have been cleared from the paddock but often very similar vegetation can be seen in nearby road and nature reserves.
In areas of the lower rainfall zone (250-350 mm annual average rainfall), soil dominated by salmon gum (Eucalyptus salmonophloia) and red morrel (Eucalyptus longicornis) is generally sodic, alkaline and affected by transient salinity. The two species can occur in close proximity, but ‘morrel soil’ is widely regarded as being extremely dispersive and, as a consequence, highly saline at depth.
In higher rainfall areas (400-600 mm annual average rainfall), soil dominated by mallet (Eucalyptus transcontinentalis, Eucalyptus spathulata) and mallee species (Eucalyptus redunca, Eucalyptus brachycorys, Eucalyptus platypus) is regarded as being sodic, dispersive and subject to seasonal waterlogging.
Eucalyptus conglobata and Eucalyptus indurata are found associated with sodic soil on the south coast.
Soil structure
In texture contrast (sand over clay) duplex soil, the sodic clays beneath the sand in the B-horizon often have columns of clay with domed tops. The domes are polyhedral with diameters of 100-400 mm, have a relatively impermeable surface skin, and either abut each other or are separated by sand lenses. These domes can often be seen in eroded duplex soil where the upper sandy layer has been stripped away.
In semi-arid environments, soil at risk of sodicity is often sandy clay that is highly alkaline and moderately saline at depth.
Soil chemistry
Alkaline soil is often easily diagnosed because of the presence of white calcium carbonate nodules in soil profiles at depths of 200-400 mm or less. In wind eroded soil, these can often be clearly seen at the soil surface. Their composition as calcium carbonate can be confirmed by the application of a weak acid (like vinegar) which will cause the rocks to ‘fizz’.
Soil function
Sodic clays are often discernible because of their inability to allow easy penetration of water. This soil will often have muddy puddles on the surface for weeks after rain.
Some sodic soil shows spatial variation in the main clay minerals present. Over long periods of time, the shrink-swell characteristics of clay minerals like smectite and illite can lead to a localised lowering of the soil surface to produce a phenomenon known as a ‘crab-hole’. Crab-holes are generally 5-15 m in diameter and may be 100-200 mm lower than surrounding soil.
Symptoms of toxicity
Transient salinity and boron toxicity are common constraints in the sodic and alkaline clay soil of the lower rainfall (250–350 mm annual average rainfall) regions of the Western Australian wheatbelt. At the scale of single plants, salt and boron have quite different symptoms in cereals.
With salinity, there is an accelerated senescence (yellowing) and then necrosis (dehydration) of leaves. This begins with the leaf tips of the oldest leaves, gradually affecting whole leaves, and ultimately whole plants. The effects are relatively similar to the normal leaf senescence associated with plant ripening. The give-away that this is not normal ripening is when the damage appears in relatively young plants.
Boron is an essential nutrient for crop growth but, when present in the soil at levels above 5 millligrams per kilogram (0.01 M CaCl2 hot extraction), it is toxic to susceptible plants such as barley. With boron toxicity, the symptoms appear as dark brown or black lesions (spots) on the leaves. In barley, all leaves, including green leaves, can show these symptoms. These lesions are sometimes confused with the symptoms of disease.
Measuring dispersion
Sodicity is often seen as synonymous with dispersive behaviour. However not all sodic soil is dispersive. Non-sodic soil can also disperse, depending on its chemistry (i.e. pH, organic carbon, type of cation, type of clay and electrolyte concentration). Measuring dispersion improves the diagnosis beyond measuring the exchangeable sodium percentage alone and enables the most appropriate amelioration or mitigation strategy to be chosen.
The modified Emerson test is a simple but useful method for rating the degree of soil dispersion and is suitable for farm-scale investigations. This test can be conducted in a laboratory or on the farm.
Laboratory methods measure dispersion by agitating the soil aggregates in deionised water and, after a set time, the dispersed clay is extracted from the solution to be dried and weighed.
Soil chemical properties including the exchangeable sodium percentage, calcium to magnesium ratio, sodium absorption ratio, stability index, electro chemical stability index and net negative charge, have also been used to predict dispersive behaviour with varying degrees of reliability. Direct measures of dispersion are recommended given that soil chemical indexes and ratios are not always reliable.
Soil chemistry
Soil at risk of dispersion is generally soil with more than 20% clay, characterised by elevated sodicity (exchangeable sodium percentage greater than 6) and elevated pH values (particularly in low rainfall areas). As a consequence of these causes of dispersion there can also be elevated salinity (EC1:5 values above 0.3 dS/m) within the subsoil. The main soluble cation is sodium (Na+), and the main anions are chloride (Cl–), and (depending on soil pH) bicarbonate (HCO3–) and carbonate (CO32-) ions. Insoluble calcium carbonate also accumulates in soil as the pH increases from 7 to 9. Elevated cation and anion concentrations in clay soil increases the apparent electrical conductivity of soil (detectable with EM38 or Dual EM).
Crops growing in affected soil may have symptoms of sodium toxicity (necrosis of the tips of young leaves, accelerated senescence of the older leaves) and symptoms of boron toxicity (black-brown spots on the leaves).
The pH of sodic soil is strongly affected by rainfall relative to evapotranspiration. In regions of high rainfall relative to evaporation (e.g. in the Lower Great Southern region of south-west of Australia) sodic soil tends to be acidic (low pH). However, the soil is more likely to be neutral or alkaline (high pH) as rainfall relative to evapotranspiration falls. In many areas of the northern and eastern wheatbelt of Western Australia, the sodic soil is alkaline.
Soil (in Australia) rated for sodicity according to exchangeable sodium percentage (ESP)
| ESP | Sodicity rating |
|---|---|
| less than 6 | non-sodic |
| 6-10 | slightly sodic |
| 10-15 | moderately sodic |
| greater than 15 | highly sodic |
From Soil Quality: 8 Sodic and Alkaline Soil (Barrett-Lennard et al. 2022).
Northcote KH and Skene JKM (1972). Australian soils with saline and sodic properties. Soil Publication. No. 27. CSIRO Aust.
Rengasamy P, North S, Smith A (2010). Diagnosis and management of sodicity and salinity in soil and water in the Murray Irrigation region. The University of Adelaide, SA. ISBN: 0 9750134 4 0
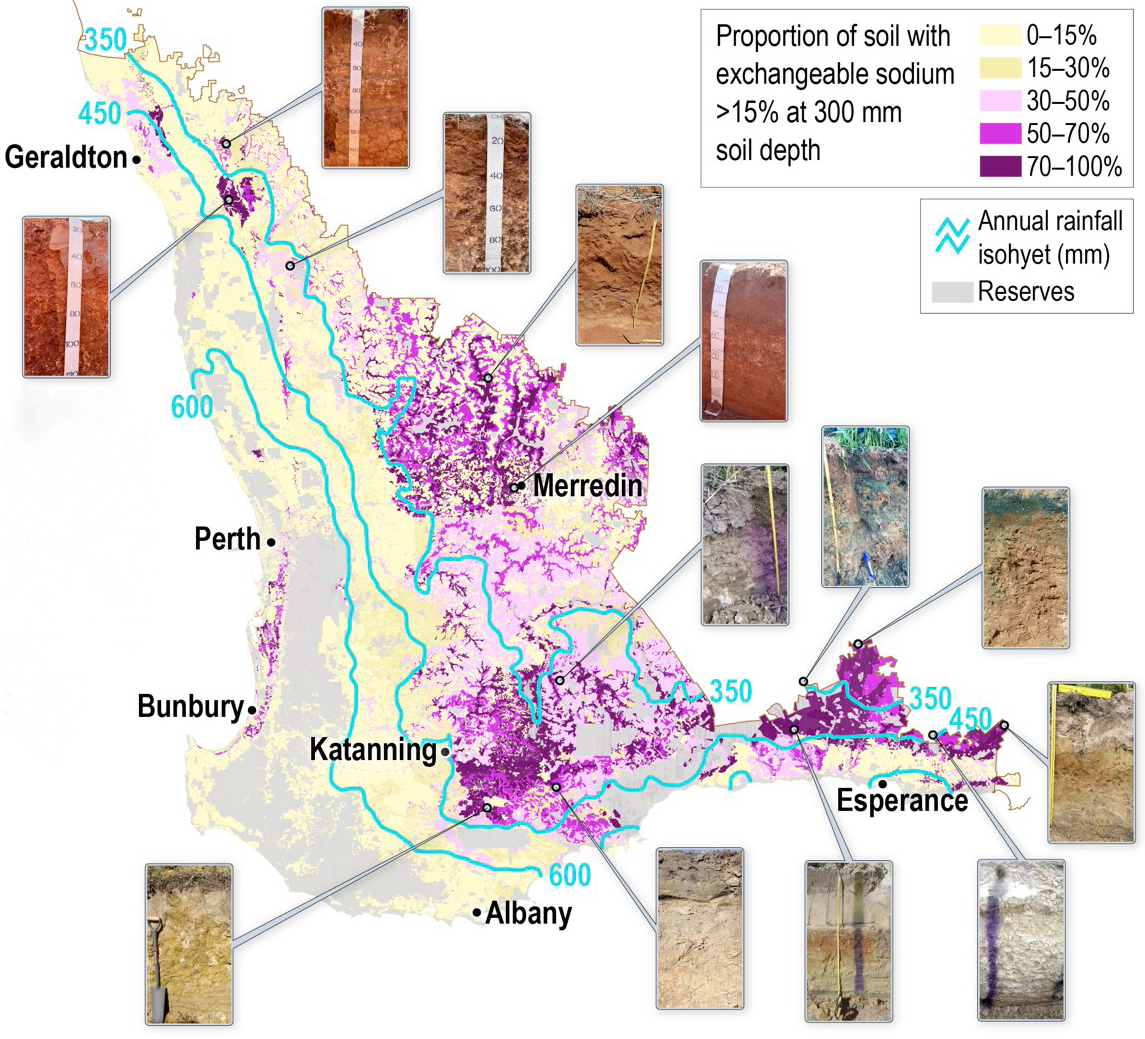
Remote sensing
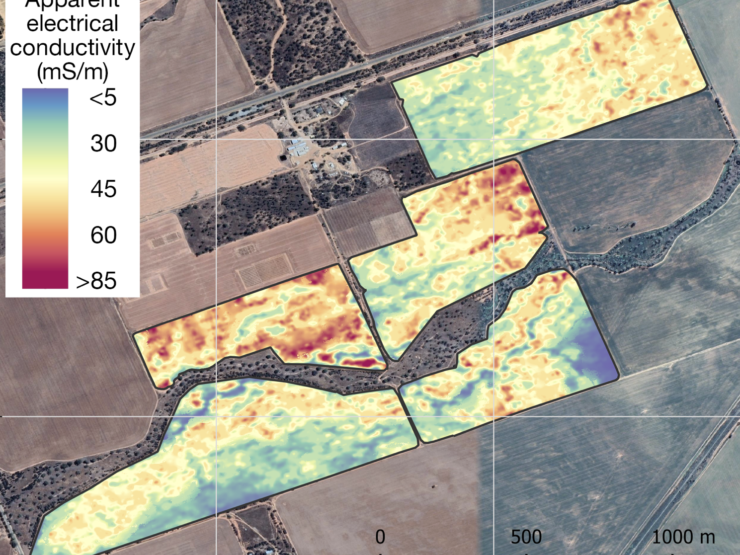
One valuable way of mapping variation in soil properties at paddock-scale is through the use of surveys produced by electromagnetic induction and radiometrics.
Electromagnetic induction surveys
A range of companies will now survey and map the variation in apparent electrical conductivity (ECa) of soil at paddock-scale. Apparent electrical conductivity readings are generally obtained with an EM38 or DualEM. Electromagnetic surveys can be used to identify areas of paddocks that might be affected by transient salinity, a major indicator of sodicity and alkalinity, and enable growers to define areas that may need to be managed differently. Apparent electrical conductivity readings greater than 60 mS/m at soil depth 0-1 m may be associated with the transient salinity caused by sodicity and alkanity.
The strength of the signal received by instruments such as EM38 and DualEM depends on the apparent electrical conductivity of the soil. The main factors affecting the apparent electrical conductivity are the salinity, clay content, and water content of the soil. Some salinity researchers have used ‘calibrated’ electromagnetic surveys to estimate the salinity of the soil solution. Calibration requires spot sampling and laboratory analysis of soil parameters to provide an understanding of the electromagnetic readings. One recent development in electromagnetic surveys has been the development of quasi-3D inversion techniques that allow for an understanding of the variation in soil conductivity in three dimensions.
Radiometric surveys
Radiometric surveys measure gamma-radiation of thorium (Th), uranium (U) and potassium (K). The concentrations of these in the soil are affected by surface soil texture, and these isotopes can be detected and mapped using vehicle-based instruments. Clay and gravel soil are associated with higher emissions of gamma-K and gamma-Th respectively, whereas sand has low gamma emissions.
Contract services
Several companies now offer contract services to conduct EM and radiometric soil surveys on a paddock-scale. Typically, an electromagnetic instrument (EM38 or DualEM) is dragged on a sled made of a non-conductive material (generally PVC) about 2 metres behind a vehicle. Readings are taken and logged every 1–2 metres in the transect row. Transects can be set at any distance apart required, but a spacing of 20 metres gives reasonable signal resolution at the paddock-scale. The collected data are cross-referenced to GPS data (latitude and longitude). These are then assembled (using mapping software) into maps of apparent electrical conductivity and maps of radiometric signatures.
Managing sodic and alkaline soil
Amelioration
The concepts for ameliorating sodic soil are well known and focus on decreasing dispersion and soil strength, increasing water infiltration, and leaching accumulated salts and toxins beyond the root zone. However, the implementation of strategies to achieve this are more complex, often requiring combinations of amendments, tillage, water harvesting and mulches.
Gypsum
Gypsum (CaSO4.2H2O) is widely used to ameliorate dispersive soils due to its high effectiveness, wide availability and relatively low cost. Gypsum causes an ‘electrolyte effect’. As gypsum dissolves there is an increase in the electrolyte concentration in the soil solution that compresses the diffuse double layer of clay platelets, resulting in clay flocculation and improved soil structure. The electrolyte concentration required to flocculate clays varies depending on their management, sodicity and mineralogy. Soil solutions with salt levels greater than 0.3–0.5 deciSiemens per metre will minimise dispersion across a wide range of exchangeable sodium percentages.
In addition to the electrolyte effect, gypsum reduces dispersion by replacing sodium (Na+) with calcium (Ca2+) ions within the diffuse double layer. Calcium ions form strong electrostatic bonds with clay surfaces primarily due to their double valency (Ca2+).
The amount of gypsum required to reduce exchangeable sodium percentage values to less than 6 within the root zone can be calculated. Often the amount of gypsum required is high (i.e. >20 t/ha) and if applied in a single application is unlikely to be profitable. Under rainfed conditions, gypsum is dissolved and leached from soil at the rate of approximately 1 tonne per hectare for every 120–130 millimetres of rainfall. To achieve a balance of electrolyte effects and improvements in calcium–sodium exchange, extensive trials show that the optimal rate for the application of gypsum is between 2 and 5 tonnes per hectare. Hence the replacement of sodium ions with calcium ions is a long-term process, resulting in sustained improvement in structural stability only with repeated applications.
Not all sodic soil is gypsum responsive. Gypsum responses are affected by a range of factors including, pH, mineralogy, electrical conductivity, co-cations (Mg2+, K+), organic carbon and the depth of the dispersive layer in the profile. The application of gypsum is likely to be highly effective in soil that is dispersive throughout the profile. However, gypsum may be less effective in duplex soil profiles where the dispersive layer is in the subsoil. Currently there is no reliable method that relates soil properties to likely yield increases from applied gypsum.
Identifying soil that is likely to respond to gypsum includes visual observations (e.g. dispersed clay in puddles), dispersion tests, chemical tests (e.g. exchangeable sodium percentage) and strip trials on targeted soil types. Electromagnetic induction can be used to map sodic soil based on clay, salt and water content. These maps can be converted into variable rate gypsum maps where zones and gypsum rates are defined by ranges of apparent electrical conductivity. Universal calibrations relating optimal rates of gypsum application relative to values of apparent electrical conductivity are currently not available as they tend to be site specific. Gypsum is invariably applied within the zones at rates up to 5 tonnes per hectare.
Lime
Calcium is the most effective ameliorant for managing dispersive soil. Apart from gypsum, calcium is also present in agricultural lime (e.g. limesand, limestone and dolomite). Compared to gypsum, lime has almost twice the concentration (by weight) of calcium. However, lime is more than 100 times less soluble than gypsum and becomes even less soluble as the pH increases beyond 7. The amount of electrolyte produced by lime is therefore considerably less than by gypsum. In low rainfall regions of the eastern wheatbelt, most sodic clays are alkaline and already contain lime in the form of carbonates and calcretes. However, in the higher rainfall region (>400 mm annual rainfall) sodic clays can be mildly acidic (pH 6–7). In these mildly acidic soil types, lime may be complementary to gypsum by providing a long-term slow-release source of calcium ions and electrolyte. The economics of using agricultural lime and gypsum together is not known as there has been no research on lime use on sodic soil in Western Australia.
A case for using lime with gypsum can be made based on data from mildly acidic soil in other States in Australia. Overall, the research suggests that where topsoil pH is less than 7, then responses to lime or combinations of gypsum and lime may be beneficial to soil structure and crop growth through the sustained release of electrolytes and calcium.
Findings from trials in eastern Australia where various combinations of gypsum and lime were used to treat dispersive soil
| Soil | Impact of lime and gypsum treatments |
|---|---|
| Chromosol (pH 5-6) Adelaide, SA | Lime increased surface macroporosity more so than gypsum, which was attributed to greater earthworm activity. However, gypsum was more effective in reducing dispersion in straw amended soil. |
| Red Sodosol (pHCaCl2 6) Condobolin, NSW | 12 years after application, lime alone had a larger impact on soil physical properties than combinations of gypsum and lime. The residual benefits on aggregate stability and hydraulic conductivity were evident where lime had been applied at 5 t/ha, whereas no residual effects from gypsum were found. |
| Grey Vertosol (pHCaCl2 6.5) Condobolin, NSW | The application of gypsum (5 t/ha) and lime (2.4 t/ha) was the most profitable treatment on cracking clay with pHca 6.5 |
| Sodic soil (pH <7) South Australia | A review of research found that where topsoil pH is less than 7, responses to lime or combinations of gypsum and lime may be beneficial to soil structure and crop growth through the sustained release of electrolyte and calcium. |
| Brown Sodosol (pHw 6-7) Peak Hill, NSW | The combination of gypsum (1 t/ha) and lime (2.5 t/ha) resulted in significant improvements in water infiltration, available water, penetrometer resistance, aggregate stability and crop yield compared to lime or gypsum alone. Most of the improvements in surface structure were attributed to the lime. |
From Soil Quality: 8 Sodic and Alkaline Soil (Barrett-Lennard et al. 2022).
Baldock JA, Aoyama M, Oades JM, Grant CD (1994). Structural amelioration of a South Australian red-brown earth using calcium and organic amendments. Soil Research 32(3): 571-594.
Bennett JM, Greene RS, Murphy BW, Hocking P, Tongway D (2014). Influence of lime and gypsum on long-term rehabilitation of a Red Sodosol, in a semi-arid environment of New South Wales. Soil Research 52(2): 120-128.
McKenzie DC, Abbott TS, Chan KY, Slavich PG, Hall DJ (1993). The nature, distribution and management of sodic soils in New-South-Wales. Soil Research 31(6): 839-868.
Naidu R , Merry RH , Churchman GJ , Wright MJ , Murray RS , Fitzpatrick RW Zarcinas BA (1993). Sodicity in South Australia – a review. Soil Research 31(6): 911-929.
Valzano FP, Murphy BW, Greene RS (2001). The long-term effects of lime (CaCO3), gypsum (CaSO4.2H2O), and tillage on the physical and chemical properties of a sodic red-brown earth. Soil Research 39(6): 1307-1331.
Organic amendments and mulches
Living and decaying organic matter within soil contributes to the creation and stability of soil aggregates. Macro-aggregate (>250 µm diameter) stability increases where plant roots and fungi/fungal hyphae enmesh and stabilise soil mineral particles and is indicative of the strong influence of soil management on root growth, physical disruption of the soil matrix and decomposition of organic matter. The stability of smaller micro-aggregates (2–20 µm) is more related to soil type and relatively independent of management, where more persistent organic substances (including microbial debris and organo-metallic complexes) form bonds with clay to stabilise. Hence, the influence of organic materials on the water-stability of aggregates has a role in preventing both slaking and dispersion.
One paradox is that the addition of organic matter to sodic soil can both increase and decrease dispersion. Shorter-term, organic material can increase the leaching of salts, resulting in greater clay dispersion. It can also increase potassium ions and increase net negative charge through the addition of soluble organic anions. However, in the longer-term, organic matter (and its derivatives) improve soil structure by binding soil particles to form water-stable aggregates. The deep placement of organic amendments in concentrated bands for example, can stimulate root growth and enhance soil porosity both within and beyond the banded zones. The resultant increase in crop yield is attributable to both improved soil structure and nutrition and may be more effective in higher rainfall years or regions, particularly where sodic clay layers are anaerobic.
Organic matter left on the soil surface can act as a mulch. Observations of increased water availability and plant production on paddocks where header rows have been evident, or where grain or hay have been left on the surface of a sodic soil may suggest lower soil evaporation and need further quantification. Crop residues and stubble retention are an important source of mulch on the soil surface as well as contributing to long-term aggregate stability, potentially providing benefits in sodic and alkaline soil. Apart from this, the management of surface organic mulches and the quantities required are currently seen as impractical in broad-scale farming systems.
Other amelioration strategies
Researchers are investigating a range of other amelioration strategies for sodic and alkaline soil. One of these is the use of elemental sulfur to acidify alkaline soil. This strategy is not currently recommended as precise guidelines are yet to be developed.
Mitigation
Management of waterlogging
Sodicity in subsoil clay can cause waterlogging, particularly in the duplex soil of landscapes of higher rainfall. Western Australia has had a long history of managing waterlogging in duplex soil through the use of banks and drains. These techniques can be used when managing waterlogging induced by sodic clays.
Tolerant crops and pasture varieties
Better crop selection is an important mitigation strategy for sodic and alkaline soil affected by transient salinity. Amongst the major crops grown in Western Australia, barley and canola are moderately tolerant to salinity, wheat is less tolerant, and legumes like field peas and lupins are quite sensitive.
Boron concentrations increase exponentially as soil pH values increase from 7 to 9. Limited datasets from Western Australia, indicate that the concentration of boron in soil is also highly correlated with soil salinity and the concentration of soluble sodium ions. Therefore, in the landscape, soil with high soil boron concentration also has high soil salinity, and these two factors cannot be disentangled.
Ideally, crops grown on sodic and alkaline soil would have high tolerance to both salinity and boron. Compared with wheat, barley is known to be more salt-tolerant, but is also sensitive to boron. However, some of the important wheat varieties used in Western Australia (e.g. Scepter and Mace) were bred in soil affected by transient salinity; and are therefore quite well adapted to soil affected by salinity, sodicity and alkaline pH.
Agronomic management
In semi-arid areas (250-350 mm annual rainfall) with sodic and alkaline soil, crops can be expected to have decreased grain yields in dry years but high grain yields in wet years. Some farmers who have sodic and alkaline soil adopt a Plan A/Plan B approach. If a season has started with excellent autumn rainfall, soil may have ample stored water; and there may also be some leaching of the salt (associated with transient salinity) into the subsoil (the salinity of the soil solution in the upper soil has therefore been decreased). In this instance, sodic and alkaline soil can be included in the seeding program. However, if there has been little or no pre-seasonal rainfall and early winter rains have been delayed, soil may remain saline; in this scenario there may be a case for removing sodic and alkaline soil from the seeding program.
Integrated management of sodic and alkaline soil
Multiple strategies exist for the mitigation and amelioration of sodic and alkaline soil.
Strategies for integrated management of sodic and alkaline soil
| Mitigation | Method | Benefits | Risks |
|---|---|---|---|
| Paddock selection | Remove highly sodic clay with transient salinity from cropping rotations in water-limiting seasons - risk of poor yield increases where soil water profiles are depleted at the beginning of the season. Low rainfall environments where hot and dry finished to the season are common are at most risk. | Minimises economic losses due to crop failure Ground cover is maintained to limit erosion and reduce surface evaporation Opportunity to control persistent weeds and diseases | Lower profits in seasons where high in-season rainfall compensates for low initial water storage |
| Crop selection | Optimise crop species and varieties to soil conditions in terms of season length, salt and boron tolerance. | Improved production and profitability Improved groundcover | Sodic and boron tolerant species or varieties selected may not be as profitable in high rainfall years |
| Input rates | Apply input (seed, fertiliser, pesticide) rates to match expected yield. | Lower variable costs of production | Yield may not achieve the potential in years of high in-season rainfall |
| Stubble and soil cover | Retain sufficient stubble or pasture cover to protect the surface soil from sealing (crusting and hardsetting) due to raindrop impact. | Improved groundcover for wind and water erosion control Reduced incidence of hardsetting and crusting Improved germination | Small risk of weed and disease carry-over |
| Amelioration | Method | Benefits | Risks |
| Gypsum application | Gypsum remains the main chemical ameliorant to improve soil structure by decreasing dispersion. Gypsum will also decrease soil pH (acidify) in highly alkaline soil. | Reduced incidence of dispersion Improved water storage and drainage Improved crop growth on gypsum responsive soil Longer term benefits from calcium replacement of sodium Decreases soil pH | Not all sodic soil is gypsum responsive Not as effective on deeper sodic layers (i.e. duplex profiles) |
| Lime application | Lime can be an option for sodic soil where topsoil pH is less than 6.5. | Calcium content of lime (40%) is higher than gypsum (23%) | Not proven as an amendment in WA Only a benefit in sodic soil with acid to neutral pH Not as soluble as gypsum - produces less electrolyte |
| Elemental sulfur application | Elemental sulfur, while improving soil structure by lowering soil pH, is not recommended due to its cost, uncertainty about best rates of application and difficulties in handling. | Decreases soil pH Converts to gypsum in calcareous soil Decreases dispersion | Rates of application are not well defined More expensive than gypsum Toxic, flammable and corrosive |
| Deep tillage | Deeper tillage can be an option where there are well defined layers that impede water and root movement. These layers may be induced (i.e. compaction) or naturally occurring (i.e. cemented layers). | Breaks up root-impeding layers Increases depth of roots and plant available water capacity Increases crop yield where yield is constrained by hard layers | No benefit where root-impeding layers are chemical (salt, B, Cl, Na) Deep tillage can bring to the surface toxic or highly unstable subsoil, which can cause prolonged crop losses Deep tillage can produce a course seedbed that limits germination Risks often greater than benefits |
| Water harvesting | Water harvesting where water is shed from the interrow to the row has consistently improved yields in research trials. Commercial adoption is dependent on the development of cheap biodegradable polymers to enhance water runoff from the interrow. | Consistent average barley yield improvements of 0.6 t/ha | Row spacing is at 375 mm Mounds need to be formed at seeding Mounds need to be sealed (plastic, polymer) to induce runoff into the seeded rows |
| Biological amelioration | Organic materials and plant root systems help to form biopores, enhancing water infiltration and drainage. Organic ameliorants stimulate root growth through additional nutrition and improved soil structure. | Potential to reduce dispersion Potential to improve soil structure Improved crop yields in higher rainfall environments | Cost of organic materials and deep placement In dry environments, risk of rapid early growth at the expense of grain yield |
From Soil Quality: 8 Sodic and Alkaline Soil (Barrett-Lennard et al. 2022).

A 'systems' approach to managing sodic and alkaline soil
A combination of crop choice, water harvesting, gypsum application, maximising soil cover and no livestock, and then maintaining the improved surface soil structure with controlled traffic, mean that the Nixon's sodic and alkaline soil is productive. Video talent: Bob and Amanda Nixon, growers at Kalannie; video production: Red Empire.
Page references and acknowledgements
Material on this page adapted from:
- Pluske W, Boggs G and Leopold M (2018). Soil Quality: 2 Integrated Soil Management. SoilsWest, Perth, Western Australia. [Access]
- Barrett-Lennard EG, Hall D, Parker W and Munir R (2022). Soil Quality: 8 Sodic and Alkaline Soil. SoilsWest, Perth, Western Australia. [Access]
Last updated July 2024.