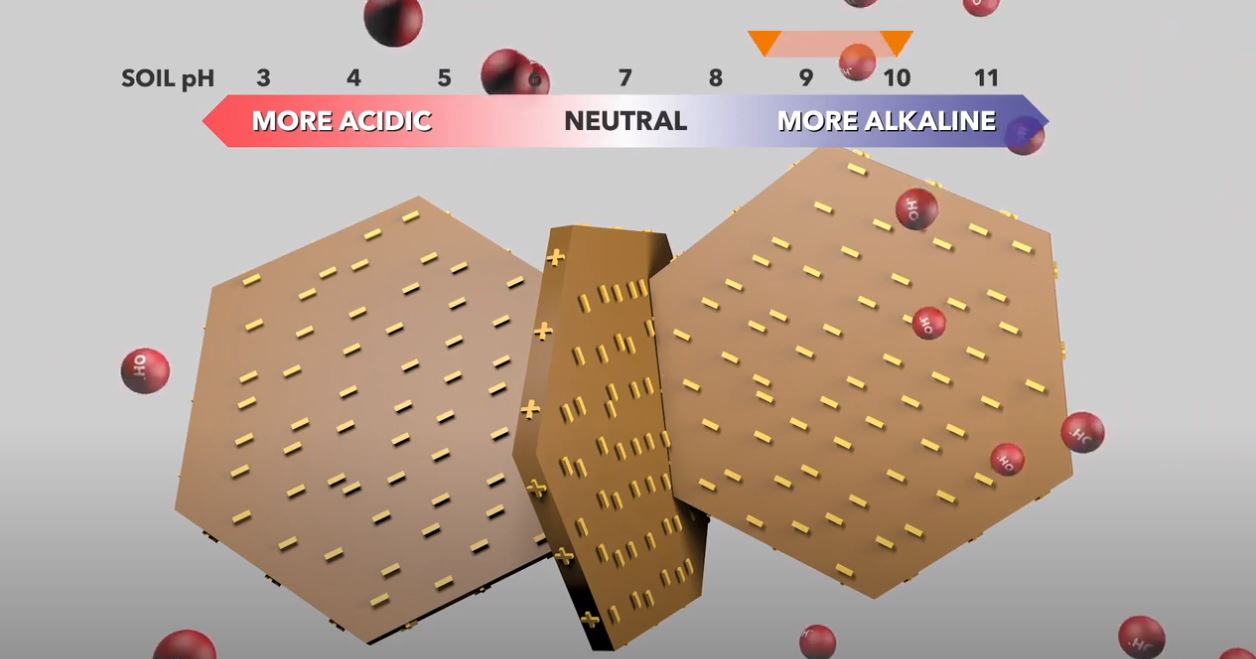
What is soil pH?
Soil pH describes the soil’s acidity or alkalinity.
Soil pH is an important indicator of chemical and biological processes that occur in soil. Extremely high or low pH can have a negative effect on the health of plants and soil biota. Although soil acidification is a natural process, it can be accelerated under agriculture. Soil acidity and soil alkalinity are significant constraints to production in many areas of Australia.
- The pH scale ranges from 1 (acidic) to 14 (alkaline) with pH 7 being neutral.
- The pH scale is logarithmic. A small decrease in soil pH will result in a large increase in acidity.
- Most plants grow best in soil with pHCaCl2 between 5.5 and 7.5 however this can vary in plant and crop species.
- The buffering capacity of soil indicates its capacity to resist changes in pH. Sandy soil is poorly buffered, and clay soil and soil high in organic matter are also highly buffered.
The pH scale
Soil pH is used to indicate the net acidity or alkalinity of soil. It is a measure of the concentration of hydrogen ions (H+) in the soil solution on a logarithmic scale. pH is measured from 1 (acidic) to 14 (alkaline), with 7 being neutral.
The pH scale is logarithmic. This means that soil with pH 4 is:
- 10 times more acidic than soil with pH 5
- 100 times more acidic than soil with pH 6
- 1000 times more acidic than soil with pH 7
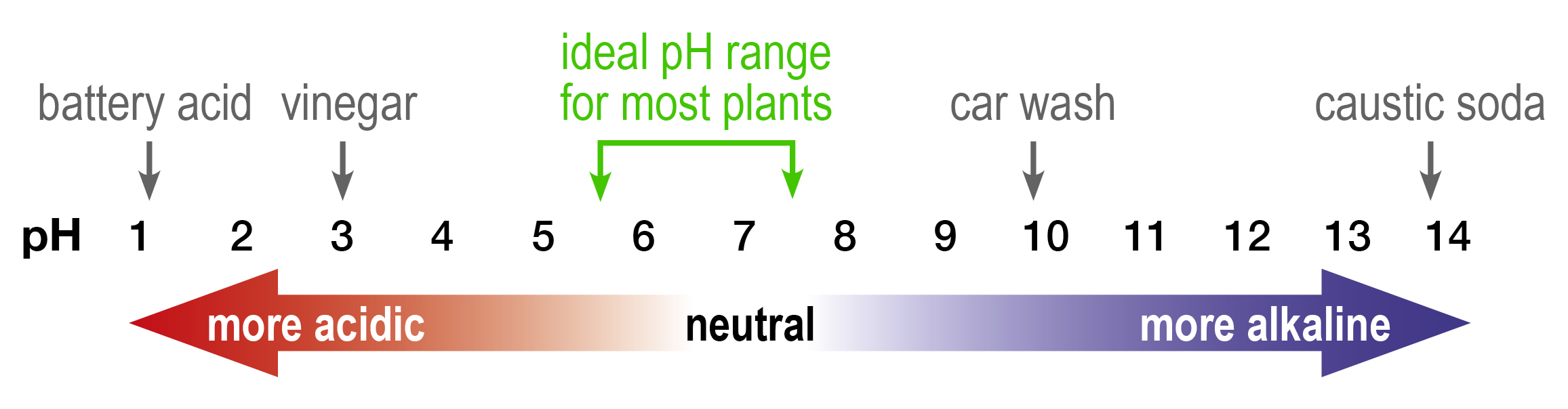
The logarithmic scale of pH is important to be aware of when managing soil pH levels as there is 10 x more acidity to neutralise with each pH unit away from the target pH level.
The chemical environment of the soil can be acid, neutral or alkaline. A soil pHCaCl2 between 6.5 and 7.5 is considered close to neutral, less than pH 6.5 slightly acid, less than pH 5.5 moderately to strongly acidic and extreme acidity associated with soils of pH less than 4.5. Acid sulphate soils (ASS) can have pH values much less than 4. Above pH 7.5, soils are considered alkaline with soil between pH 7.5 and 8 often associated with free calcium carbonate (lime equivalent) and above pH 8.5, sodium salts.
The movement of strongly acid balancing ions such as sulphate or nitrate through the soil generates excess acidity and the accumulation of strongly basic ions (i.e. calcium and sodium) generates alkalinity. Australian soils range from pH 3 (peat bogs) to pH 10 (arid desert soils), though most agricultural soils are in the pH range 4.5 – 9. A pH range of 5 – 7 is ideal for most plants, with some showing a preference for more acid soils, and others preferring alkaline soils. Acidity develops as the concentration of hydrogen ions increases in soil and as a result soil pH declines.
The pH can vary significantly between different soil layers. For example sandy topsoil with a pH of 5 may have clay subsoil with a pH of 8 or sandy subsoil where pH continues to decline. It is important to measure soil pH in different horizons of the soil to ensure appropriate management.
Areas of high rainfall such as those on the coast and mountains often have more acidic soils compared to more arid areas, due to the more intense leaching in wetter climates. Other soils contain large amounts of carbonates (limestone derived from marine sediments) or alkaline salts in their groundwater – these soils can by very alkaline, even in high rainfall areas.
Factors influencing soil pH
Soil processes that lead to a decrease in hydrogen (H) ions will increase pH. An increase in free hydrogen ions will lower pH.
Soil acidification is a natural process, but it can be accelerated under agriculture. Although soil alkalinity affects soil processes and management, it is typically the acidification of soil that is of more concern for management.
Acid soil formation occurs on naturally acidic parent material such as mountain peat, whilst alkaline soils tend to be associated with naturally alkaline parent material such as limestone. Soil types can have a typical pH range however it is always important to conduct a soil test for pH to confirm.
The pH buffering capacity of a soil indicates the capacity of the soil to resist pH change. The amount of soil organic matter and clay in soil influences its buffering capacity by providing charged exchange sites where excess ions can be held or exchanged. Highly buffered soil is slower to acidify but requires more agricultural lime to lift pH when it does acidify. Clays are generally better buffered than loams, which in turn are better buffered than sands. Sands are also less able to retain nutrients against leaching compounding the acidification rate if fertiliser is applied inefficiently. Poorly buffered sandy soil types comprise more than 40% of the agricultural land in the south-west of Western Australia. The naturally acidic peaty sands of the south coast have a high buffering capacity and would require more lime to increase pH than other soil in the region.
Nitrification is the conversion of ammonium to nitrate. During this process, hydrogen ions are produced and contribute to the release of aluminium (Al) ions and displace calcium (Ca), magnesium (Mg) and potassium (K) ions – contributing one unit of acid to the soil for each unit of nitrogen mineralised.
Nitrogen
Ammonium ions are positively charged and so are retained in soil by negative surface charge sites present on organic matter and clay. Negatively charged nitrate ions are generally not retained by soil and are subject to leaching. Leaching of nitrate nitrogen , originally applied as ammonium-based fertilisers, out of the root zone will accelerate acidification. Ammonium sulphate and mono-ammonium phosphate (MAP) are the most acidifying nitrogen fertilisers, followed by di-ammonium phosphate (DAP). Ammonium nitrate and urea acidify at a slower rate, whilst calcium nitrate does not result in net acidification.
Sulfur
When sulfur is added to soil it combines with water and oxygen to form sulfuric acid and can be used to lower soil pH in alkaline soils with no free lime but when added to soils with free lime, can increase the level of gypsum salts.
Phosphorous
Phosphate fertilisers are not directly acidifying, but do indirectly add to soil acidity by improving plant growth and stimulating nitrogen fixation. The increase in the nitrogen cycling, particularly under legume pasture phases and grazing systems can help lead to accelerated soil acidification.
If the supply of nitrate is less than plant uptake, then the potential acidification risk is low. If nitrate supply is greater than plant demand then nitrate can be leached, resulting in more rapid soil acidification. Grain crops typically acidify the soil faster than pastures, due to their inefficient use of nitrate. In contrast, annual and perennial pastures are able to establish earlier and have a longer growth phase, increasing nitrate uptake and reducing the rate of acidification. The rate of acidification is typically higher where grain legumes are used in the rotation and is dependant on the frequency of legume use, as well as soil factors such as organic matter and soil type.
Acid sulfate soils are formed by the introduction of air into either
- soil rich in non-oxidized sulfidic materials which form in waterlogged saline sediments with a supply of easily decomposed organic matter, or
- sediments containing the mineral pyrite.
The increasing amount of acid production exceeds the soil’s ability to neutralise it, resulting in the production of sulfuric acid and a soil pH below 4.
Acid deposition from industrial atmospheric pollutants such as sulphur dioxide and land contamination by acidic pollutants can contribute to increasing soil acidification.
Irrigation with alkaline bore water which contain carbonate salts will increase soil pH, as will upward movement of an alkaline water table.
Lime is commonly used as a soil ameliorant on acid soils to increase soil pH. The input of carbonate salts via alkaline dust in arid regions can increase soil alkalinity.

Assessing soil pH
Indications of an acidic soil
< pH 5.5 (CaCl2)
- Nutrient deficiencies (phosphorus, magnesium, calcium)
- Reduced boron and molybdenum availability
- Aluminium or manganese toxicity
- Failure of legumes to nodulate – this results in nitrogen deficiency, indicated by reddening of stems in pasture legumes, or yellowing and death of oldest leaves on grain legumes
- Poor root growth (stubby roots and few fine roots)
- Reduced microbial activity
- Presence of acid sulphate soils indicated by low pH readings (pH < 4)
Indications of an alkaline soil
> pH 8.3 (CaCl2)
- High pH can indicate the presence of calcium carbonate, high sodicity or the presence of toxic compounds like sodium carbonate
- Surface sealing and crusting problems due to excess sodium
- Zinc deficiency
- Boron toxicity
- Reduced microbial activity
- Increased salt concentration
Measuring soil pH
Soil pH can be measured in soil extracts obtained with deionised water or a dilute calcium chloride (CaCl2) solution. Soil samples should be analysed by a laboratory accredited with the Australasian Soil and Plant Analysis Council Inc (ASPAC). It is standard practice in Western Australia for soil pH to be measured in a weak calcium chloride solution (1 part soil to 5 parts 0.01 M calcium chloride, CaCl2).
Soil pH values measured in CaCl2 solution are closer to the true soil pH in the field and give a lower value than pH measured in water (pHw). For most soils, pH values in water are between 0.5 and 1.0 pH unit higher than values in CaCl2.
Both pH in CaCl2 and water are often reported in standard soil tests. When comparing results or monitoring pH change over time, it is important to be consistent with the same measurement. Where required, adjust to a pH value in CaCl2 to allow a comparison with the recommended target values. An approximation for acidic soil is: pH CaCl2 = pHw – 0.7
On-farm assessment of soil pH
While laboratory testing provides the most accurate measurement of soil pH, on-farm testing can give you a good idea of areas that require professional sampling and testing.
Garden soil pH test kits
Soil pH test kits that use indicator solutions and colour matching are probably the most convenient way of on-farm testing. The test kits are relatively inexpensive, easy to use and available from hardware stores. The results are subjective but will give you an estimate of pH and an indication of whether you have an acidity or alkalinity constraint that needs further investigation. These test kits estimate a water pH, so you need to subtract 0.7 from the result to approximate pH measured in calcium chloride solution.
Spraying universal pH indicator
Spraying the face of a soil pit with universal pH indicator is a quick way of assessing the pH down your soil profile to see if you have subsoil acidity or a layer of acidic soil and the depth of that. You can use the indicator solution from garden pH test kits (do not dilute) in a small pump-up sprayer.
This technique requires a little practise. Before you start, brush away any topsoil that has fallen down the pit face. The pit face needs to be damp—if it is dry, you can spray it with water but make sure the water doesn’t run down the pit face. Make sure the sprayer is well pressurised and position the spray nozzle far enough away from the pit face so that the indicator solution is applied as a fine mist. Spray the profile in a continuous motion so you get an even coating, but not so much that it appears wet. A couple of light applications are better than one heavy one.
You can use the colour indicator card from the test kit to get an idea of pH. There is no need to use the white, barium sulphate powder, which simply assists in showing up the colour changes. It is more helpful in soil types with less contrast between the indicator colour change and soil colour. In brief, green indicates good pH for most plants, orange is too acidic and purple is alkaline. Any lime you’ve applied will appear purple, which is normal and not a problem, however if the whole profile is purple, your soil is probably too alkaline and it is worth investigating further for commonly co-occurring constraints, such as sodicity and transient salinity. If you find an acidity problem, you will still need to do thorough pH sampling and laboratory testing to work out an appropriate liming regime.

Spraying universal pH indicator.
From Soil Quality: 4 Soil Acidity (Gazey et al. 2019). Video: Chris Gazey, DPIRD; editing: Science with Style.
Hand-held pH probes
A variety of hand-held pH probes are available from scientific equipment suppliers and come with detailed instructions. In addition to pH, many measure electrical conductivity, total dissolved solids, salinity and temperature. Hand-held probes can measure pH in a solution of soil and water or calcium chloride. When field testing soil pH, it is usually more convenient to use deionised or distilled water (1 part soil to 5 parts water) instead of calcium chloride solution and so the results will need to be converted by subtracting 0.7. This method is not a replacement for laboratory testing of soil pH.
Impact of soil pH
Plants and soil microorganisms generally prefer a soil pHCaCl2 range between 5 and 7 although there are tolerances outside this range. In soils with pHCaCl2 below 5.5, acid-sensitive agricultural plants are adversely affected and the risk of subsoil acidification increases. Acidity can build up gradually and may not be noticed if agronomic management is optimal – yet yield penalties of 20-30% may be occurring. Subsurface soil acidity can have as much effect on plant growth as surface acidity, but is more difficult and costly to treat.
Toxicity
Boron concentrations increase exponentially as soil pH values increase from 7 to 9. Limited datasets from Western Australia, indicate that the concentration of boron in soil is also highly correlated with soil salinity and the concentration of soluble sodium ions. Therefore, in the landscape, soil with high soil boron concentration also has high soil salinity, and these two factors cannot be disentangled.
In strongly acid soils (less than pH 4.0 to 5.0), aluminium and manganese may become available in sufficient quantities to become toxic, inhibiting root growth and reducing crop yields. An increase in the uptake of toxic heavy metals such as cadmium can also occur as the soil becomes more acidic. Aluminium toxicity is the common constraint in WA acid soil.
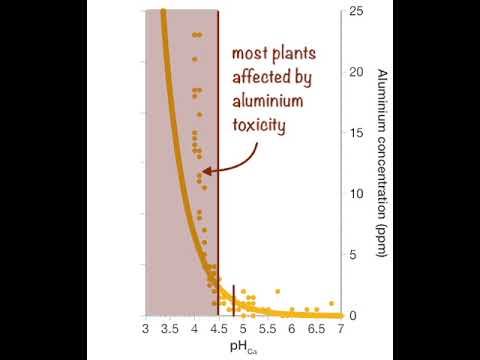
Relationship between soil pH and concentration of hydrogen ions and aluminium.
A small decrease in soil pH can result in a large increase in soil acidity and aluminium content. From Soil Quality: 4 Soil Acidity (Gazey et al. 2019). Animation: Science with Style; data: Precision SoilTech; voice talent: Ed Barrett-Lennard, DPIRD.
Nutrient deficiency
Nutrient deficiencies that affect plant growth occur at both low and high pH.
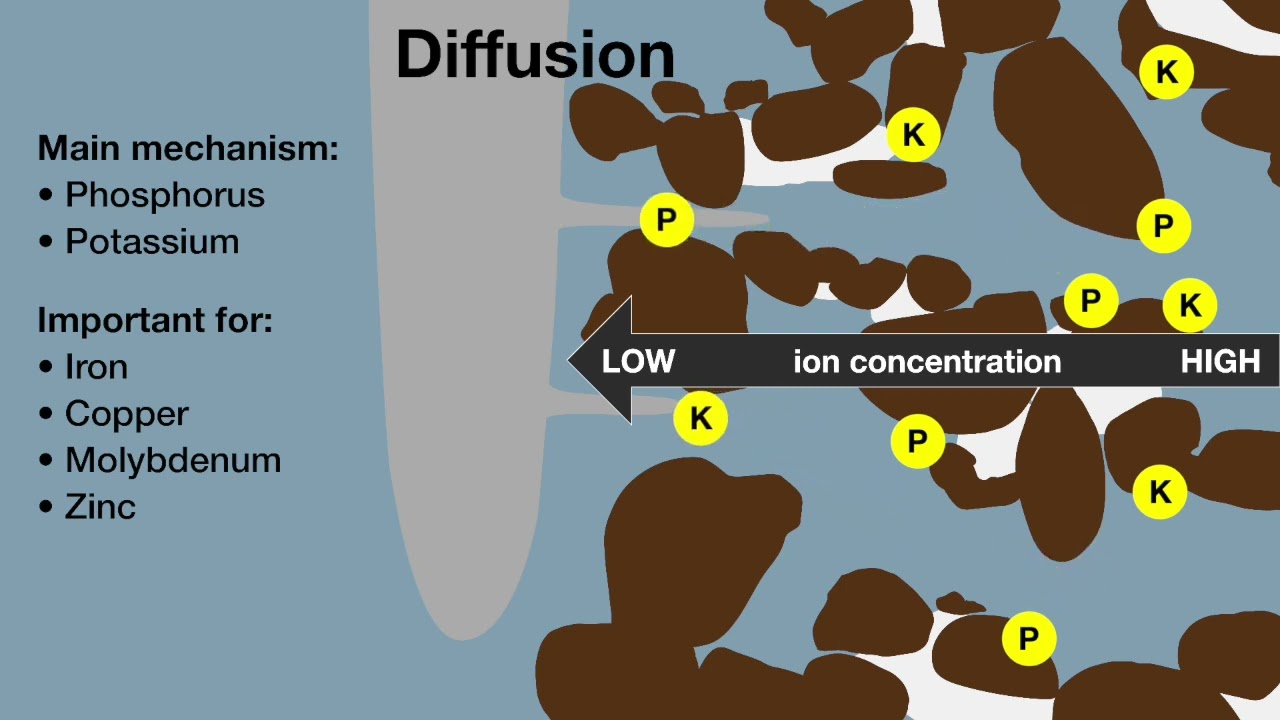
In very acid soils all major plant nutrients (nitrogen (N), phosphorus (P), potassium (K), sulphur (S), calcium (Ca) and magnesium (Mg)) and also trace element molybdenum (Mo), may be unavailable to plants, or only available in limited quantities.
In moderately to highly alkaline soil (pHW >8) micronutrients, including iron (Fe), manganese (Mn), zinc (Zn) and copper (Cu) become less available to plants because the ionic compounds they form at higher pH have decreased solubility compared to those formed at lower pH. Of the major nutrients, calcium (Ca), magnesium (Mg) and nitrogen (N) are also less available.

Water use efficiency
The ability of plants to use subsoil moisture may be limited due to poor root exploration in a highly acid or alkaline subsoil. This can result in higher leaching rates, loss of nutrients and increased deep drainage.
Subsurface acidification
If left unmanaged, increasing soil acidity on the surface can contribute to more rapid acidification of subsoil (10-30 cm depth). Susceptible soil types include the deep sands, sandy earths, gravels and duplex soils with low clay and organic carbon content and low pH buffering capacity. Subsurface acidity is more difficult to treat due to the slow movement of neutralising materials such as lime and base rich organic matter down the soil profile. It is much cheaper to avoid the risk of subsurface acidity by keeping the surface soil at pH 5.5 or more.
Biological activity and function
Biological activity is depressed in acid soils, slowing soil organic matter decomposition. Soil acidity affects the survival and persistence of bacterial groups such as rhizobia which are responsible for nitrogen fixation, slows soil processes such as nitrification , and results in fungi becoming more dominant. Earthworm and termite populations decline in acid soils.
Other
Extreme acidification can result in poorly structured or hard-setting topsoils, as well as increasing the risk of soil erosion caused by poor ground cover. Soils may also acidify to the point where acid, nutrients, sediment and heavy metals are exported and impact nearby inland waters.
Managing soil pH
Soil tests should be used to monitor paddocks showing poor growth for surface and subsoil pH, testing the same area each time to check any changes in soil that occur over time.
Soil acidification is an inevitable process unless sufficient alkalinity is returned to the soil to balance the acidification caused by nitrogen fertilisers, legumes, and product removal (grain, hay, meat, etc). Acidification can be slowed by liming, changing land use, crop mix and management of the crop. Tolerant crops can be grown within a management program.
In alkaline soils, choose a crop that is naturally tolerant of alkaline conditions. Although it is possible to acidify a strongly alkaline soil by applying acid generating nitrogen fertilisers (e.g. ammonium sulphate, monoammonium phosphate [MAP]), this is usually a slow process, especially if the soil has a high buffering capacity.
Page references and acknowledgements
Material on this page adapted from:
- Hoyle FC (2007). Soil Health Knowledge Bank.
- Pluske W, Boggs G and Leopold M (2018). Soil Quality: 2 Integrated Soil Management. SoilsWest, Perth, Western Australia. [Access]
- Gazey C, Azam G, Clausen J and Rengel Z (2019). Soil Quality: 4 Soil Acidity. SoilsWest, Perth, Western Australia. [Access]
- Barrett-Lennard EG, Hall D, Parker W and Munir R (2022). Soil Quality: 8 Sodic and Alkaline Soil. SoilsWest, Perth, Western Australia. [Access]
Last updated July 2024.